Diazonium Chemistry with a Twist
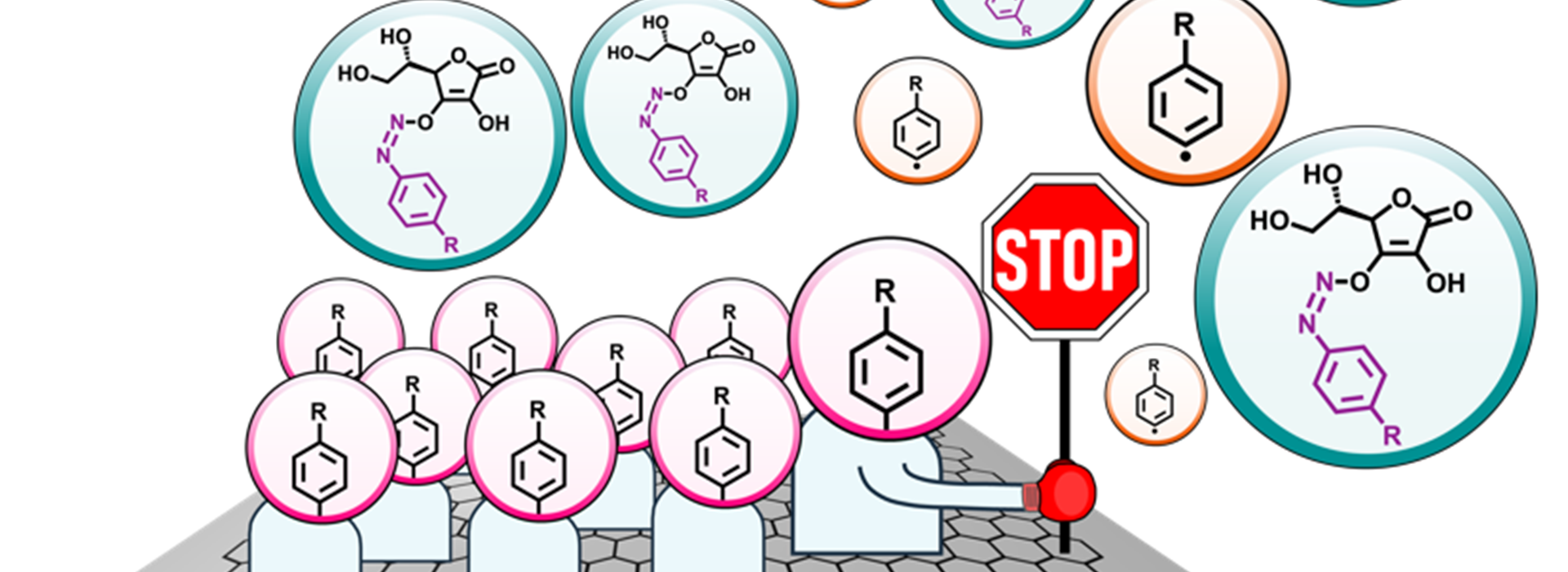
The chemistry of carbon surfaces has regained traction in recent years in view of its applicability towards covalent modification of a variety of (2D) materials. A general requisite is the formation of a dense and well-defined monolayer of aryl groups covalently bound to the surface. Given the use of reactive chemistries however, it is often not easy to achieve precise control over the monolayer growth while maintaining high grafting densities. Here we present a straightforward experimental protocol for the fabrication of well-defined covalent monolayers onto the surface of graphite. Using a combination of surface analytical tools, we demonstrate that the ascorbic acid mediated dediazoniation of aryldiazonium salts leads to selflimiting growth of monolayers with high grafting densities. The aryl radicals preferentially attach to the basal plane of the substrate and once the surface is covered with a covalent monolayer, the surface reaction does not proceed further to an appreciable extent. The layer thickness of the covalent films was measured using atomic force microscopy whereas the grafting efficiencies were assessed using Raman spectroscopy. The chemical composition of the grafted films was studied using X-ray photoelectron spectroscopy whereas scanning tunneling microscopy provided nanometer scale insight into the structure of the covalent films. Mechanistic aspects of the process are also discussed. The self-terminating chemistry described here is a new addition to the synthetic armory for covalent modification of materials and sets a strong foundation for achieving precise nanoscale control over the covalent functionalization process.
Read more about it in this paper
To enable comments sign up for a Disqus account and enter your Disqus shortname in the Articulate node settings.


